Borophene new material inspires from Graphene
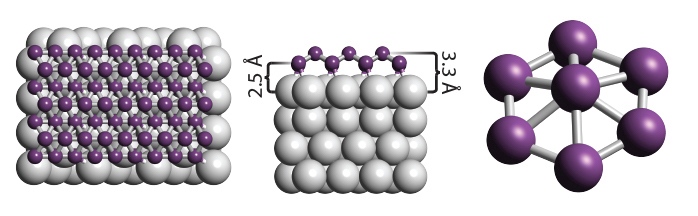
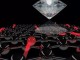
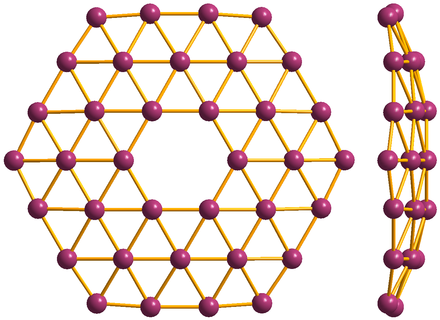
Borophene is a honeycomb of boron atoms (purple), with each hexagon capped by another boron atom
Researchers at Northwestern University have unveiled an intriguing new 2D like graphene called borophene, It’s sheet of boron just one atom thick with a graphene-like honeycomb arrangement, but with an extra boron atom placed on top of each tessellated hexagon. Borophene has not yet been isolated as a free-standing sheet, but there are already indications that some of borophene’s properties could match if not surpass, those of graphene and other 2D materials.
Now, according to researchers borophene’s properties could potentially exceed those of graphene and other, similar materials in the 2D nanomaterial family, “Borophene is just beginning to be studied,” said Mark Hersam, Northwestern University’s materials scientist, in the press release. “It’s good to get it out there so the field as a whole can take it on—there’s so much to be done with it.”
At the atomic cluster scale, pure boron is markedly similar to carbon, forming simple planar molecules and cage-like fullerenes. Theoretical studies predict that two-dimensional (2D) boron sheets will adopt an atomic configuration similar to that of boron atomic clusters. We synthesized atomically thin, crystalline 2D boron sheets on silver surfaces under ultrahigh-vacuum conditions. Atomic-scale characterization, supported by theoretical calculations, revealed structures reminiscent of fused boron clusters with multiple scales of anisotropic, out-of-plane buckling. Unlike bulk boron allotropes, borophene shows metallic characteristics that are consistent with predictions of a highly anisotropic, 2D metal.
The researchers grew borophene on silver, by evaporating boron atoms from a solid boron rod at temperatures of 450ºC to 700ºC inside an ultrahigh-vacuum chamber. While boron itself is a poor electrical conductor, the scientists found that borophene is actually fully metallic – which is extraordinary amongst other 2D materials (that are usually semiconductors). Unprotected samples oxidized in a few hours, but a silicon covering kept them stable for several weeks.
Transferring free-standing borophene to an insulating substrate would allow researchers to accurately measure its conductivity, for example. But that’s far from trivial: “Boron reacts with almost everything around it,” says Hermann Sachdev, an inorganic chemist at the Technical University of Kaiserslautern in Germany.
Boron’s reactivity may turn out to be an advantage, however, because borophene could be readily modified with other chemical groups or sandwiched between other materials to fine-tune its properties. Boron and many of its compounds are also extremely hard, so if borophene’s chemical exuberance can be controlled it might be easier to handle than more fragile flatlanders such as silicene or germanene (2D sheets of silicon and germanium, respectively).
Now the goal is to demonstrate a free-standing sheet of borophene, which would allow researchers to accurately measure properties like conductivity. It seems, however, that this is far from being easy, as boron is highly reactive to substances around it. This tendency to react may eventually turn out to be an advantage, because borophene could be readily modified with other chemical groups or sandwiched between other materials to fine-tune its properties. Boron and many of its compounds are also extremely hard, so it might be easier to handle than more fragile 2D materials like silicene or germanene.
The scientists suggest that borophene has a higher electron density than graphene, which raises the possibility that cooled borophene could act as a superconductor, carrying electrical charge with no resistance.