Team of scientists make new graphene ‘patchwork’ predicted by CAD
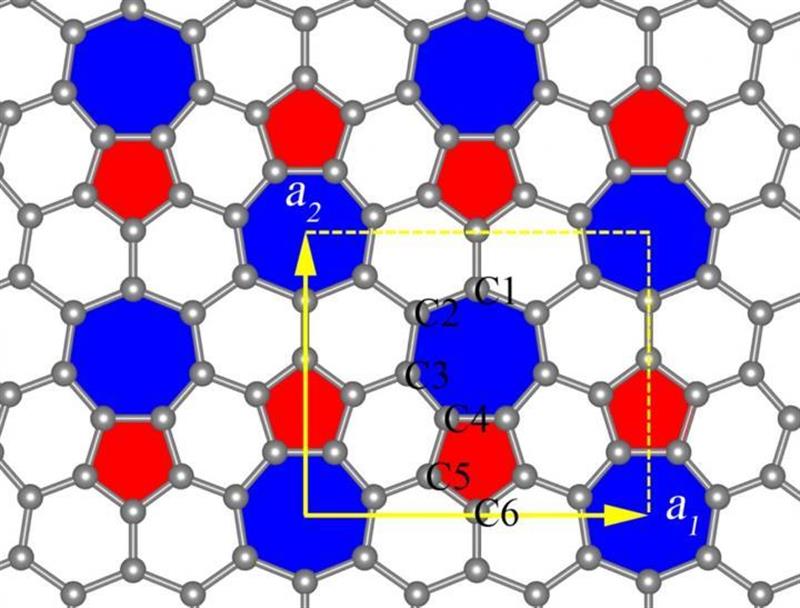
Phagraphene consists of penta-, hexa- and heptagonal carbon rings. Its name comes from a contraction of Penta-Hexa-heptA-graphene
A group of scientists from Russia, the USA and China, the leader of scientists team Artyom Oganov from the Moscow Institute of Physics and Technology (MIPT), using computer-generated simulation have predicted the existence of a new two-dimensional carbon material, scientists make new graphene “patchwork” analogue of graphene called phagraphene. The results of their investigation were recently published.
“Unlike graphene, a hexagonal honeycomb structure with atoms of carbon at its junctions, phagraphene consists of penta-, hexa- and heptagonal carbon rings. Its name comes from a contraction of Penta-Hexa-heptA-graphene,” says Oganov, head of the MIPT Laboratory of Computer Design.
Graphene is a single atomic layer of sp2-hybridized carbon arranged in a honeycomb lattice. Defect-free graphene is the strongest material in the world, but current techniques to synthesize graphene sheets large enough to use in applications invariably produce grain boundary defects. Grain boundary defects can be likened to the seams in patchwork quilts, made of pieces of fabric that have been sewn together.
Phagraphene is expected to share many of the same properties with graphene. The researchers say that in phagraphene, due to the different number of atoms in the rings, some properties are predicted to be different and that it would be interesting to see where it might be useful.
Due to its two-dimensional structure, graphene has absolutely unique properties. Most materials can transmit electric current when unbound electrons have an energy that corresponds to the conduction band of the material. When there is a gap between the range of possible electron energies, the valence band, and the range of conductivity (the so-called forbidden zone), the material acts as an insulator. When the valence band and conduction band overlap, it acts a conductor, and electrons can move under the influence of electric field.
In graphene each carbon atom has three electrons that are bound to electrons in neighboring atoms, forming chemical bonds. The fourth electron of each atom is “delocalized” throughout the whole graphene sheet, which allows it to conduct electrical current. At the same time, the forbidden zone in the graphene has zero width. If you plot the electron energy and their location in graph form, you get a figure resembling an hour glass, i.e. two cones connected by vertices. These are known as Dirac cones.
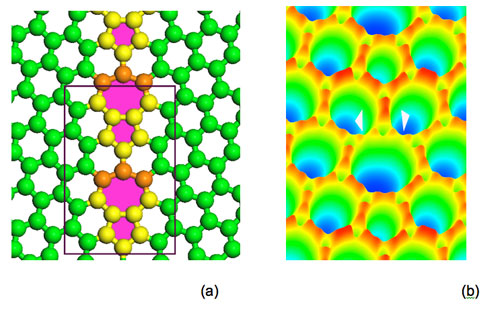
Phagraphene appear how to look in different CAD structuration
Due to this unique condition, electrons in graphene behave very strangely: all of them have one and the same velocity (which is comparable to the velocity of light), and they possess no inertia. They appear to have no mass. And, according to the theory of relativity, particles traveling at the velocity of light must behave in this manner. The velocity of electrons in graphene is about 10 thousand kilometers a second (electron velocities in a typical conductor vary from centimeters up to hundreds of meters per second).
Phagraphene, discovered by Oganov and his colleagues through the use of the USPEX algorithm, as well as graphene, is a material where Dirac cones appear, and electrons behave similarly to particles without mass.
“In phagraphene, due to the different number of atoms in the rings, the Dirac cones are ‘inclined.’ That is why the velocity of electrons in it depends on the direction. This is not the case in graphene. It would be very interesting for future practical use to see where it will be useful to vary the electron velocity,” Artyom Oganov explains.
Phagraphene possesses all the other properties of graphene that allows it to be considered an advanced material for flexible electronic devices, transistors, solar batteries, display units, and many other things.